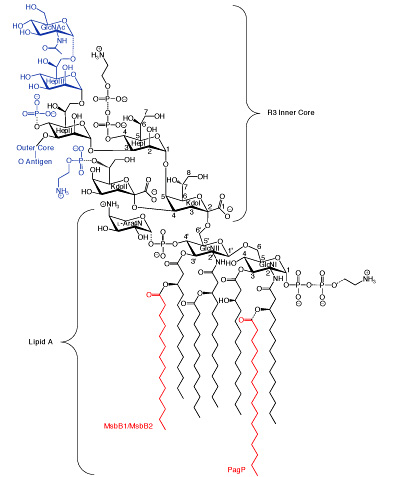
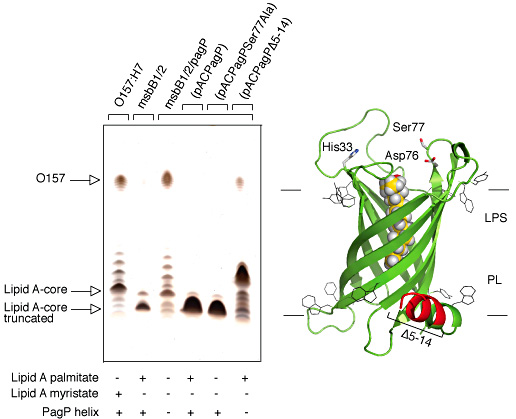
Our research on the structure and function of membrane proteins attacks one of the most important and challenging problems in contemporary biochemistry. Our most recent work has been focused on an outer membrane enzyme PagP from pathogenic Gram-negative bacteria. PagP transfers a palmitoyl group from a phospholipid molecule to the lipid A (endotoxin) component of lipopolysaccharide (LPS). This simple modification provides bacterial resistance to host antimicrobial peptides and attenuates the inflammatory response signaled through the human toll-like receptor 4 pathway. By understanding how the structural and dynamic properties of PagP relate to its biological function, we hope to design inhibitors that might be useful for the treatment of infections, and to synthesize novel endotoxin antagonists for the treatment of septic shock.
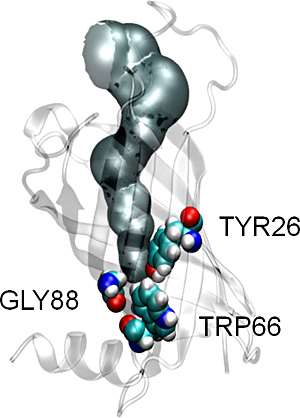
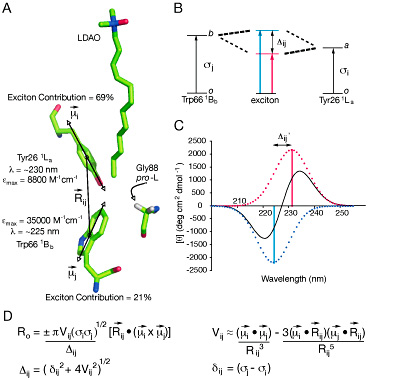
The structural basis of lipid acyl-chain selection by membrane-intrinsic enzymes is poorly understood because most integral membrane enzymes of lipid metabolism have proven refractory to structure determination; however, robust enzymes from the outer membranes of Gram-negative bacteria are now providing a first glimpse at the underlying mechanisms. The methylene unit resolution of the phospholipid:lipid A palmitoyltransferase PagP is determined by the hydrocarbon ruler, a 16-carbon saturated acyl-chain-binding pocket buried within the transmembrane β-barrel structure. Substitution of Gly88 lining the floor of the hydrocarbon ruler with Ala or Met makes the enzyme select specifically 15- or 12-carbon saturated acyl-chains, respectively, indicating that hydrocarbon ruler depth determines acyl-chain selection. However, the resolution of Gly88Cys PagP does not diminish linearly because it selects both 14- and 15-carbon saturated acyl-chains. We discovered that an exciton, emanating from a buried Tyr26-Trp66 phenol-indole interaction, is extinguished by a local structural perturbation arising from the proximal Gly88Cys PagP sulfhydryl group. Site-specific S-methylation of the single Cys afforded Gly88Cys-S-methyl PagP, which reasserted both the exciton and methylene unit resolution by specifically selecting 13-carbon saturated acyl-chains for transfer to lipid A. Unlike the other Gly88 substitutions, the Cys sulfhydryl group recedes from the hydrocarbon ruler floor and locally perturbs the subjacent Tyr26 and Trp66 aromatic rings. The resulting hydrocarbon ruler expansion thus occurs at the exciton’s expense and accommodates an extra methylene unit in the selected acyl-chain. The hydrocarbon ruler-exciton juxtaposition endows PagP with a molecular gauge to probe the structural basis of lipid acyl-chain selection in a membrane-intrinsic environment.
back to top
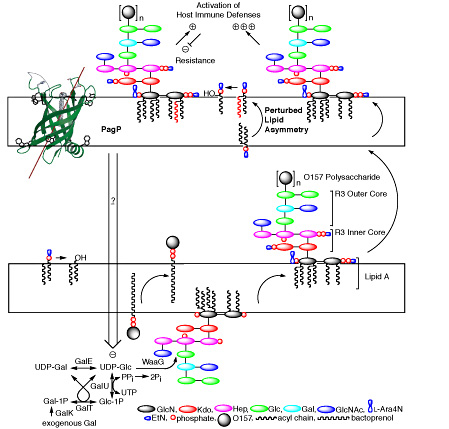
The enterobacterial outer membrane is an asymmetric lipid bilayer in which LPS exclusively lines the external leaflet while phospholipids line the inner leaflet. The asymmetric lipid organization provides a permeability barrier to hydrophobic antibiotics and detergents encountered in the natural and host environments. Although hydrophobic antibiotics can freely permeate through phospholipid bilayers, negative charges in LPS are bridged by Mg2+-ions to create tight lateral packing interactions that largely prevent permeation. Perturbations of outer membrane lipid asymmetry are thought to be associated with the migration of phospholipids into the external leaflet to create localized rafts of phospholipid bilayers, which render bacteria susceptible to hydrophobic antibiotics. Additionally, the LPS O-antigen can provide bacterial resistance to serum by preventing deposition of the complement cascade membrane attack complex.
back to top
PagP structure and dynamics demonstrate that the palmitate recognition pocket, known as the hydrocarbon ruler, is only accessible from the outer membrane external leaflet and, thus, requires aberrant translocation of phospholipids into the external leaflet. Indeed, PagP remains dormant in the outer membrane until lipid redistribution associated with a breach in the permeability barrier directly triggers PagP activity. Lipid A palmitoylation is an appropriate adaptive response, because it helps to restore the permeability barrier. However, we have also shown that outer membrane activation of PagP can exert control on cytoplasmic enzymes of LPS core oligosaccharide structure that leads to a failure in O-antigen attachment. In this case, PagP does not require its catalytic machinery, but it depends instead on a periplasmic amphipathic α-helix.
back to top
The physical separation between the outer membrane and the cytoplasm dictates that PagP must be functioning as a sensory transducer, which is activated by a breach in the outer membrane permeability barrier. Our findings indicate that outer membrane activation of PagP not only triggers lipid A palmitoylation, but also separately triggers signal transduction across three cellular compartments. Discovering the downstream signaling components that respond to the activated-state of PagP will provide fertile ground for future research.
back to top