Studies on Mammalian Adenosine Kinase and Related Enzymes
Adenosine kinase (AK) is a key enzyme responsible for maintaining the intracellular concentrations of adenosine (Ado), which shows potent cardioprotective and neuromodulatory activities. Many different types of mutants affected in AK have been isolated in my lab. The AK gene in mammalian cells is very large and the ratio of noncoding to coding sequence for this gene (>500) is highest of all known genes in mammalian cells. The AK gene in mammalian cells is also linked in a head-to-head manner with the gene for the mu3A adaptor protein, which is affected in the Herman-Pudlak syndrome. Both these genes are transcribed from a single bi-directional promoter. Many of the AK mutants that we have isolated contain large deletions in the AK gene some of which extends into the neighboring mu3A gene. Other mutants involve specific point mutations affecting the biochemical properties of the enzyme. Another novel property of AK, which it shares with a few other enzymes, that we have discovered is that its activity in different organisms is completely dependent upon the presence of pentavalent ions. Our work has led to identification of many novel compounds, which compete with pentavalent ion and act as either activators or inhibitors of AK. The inhibitors of AK are of great interest due to their potential cardioprotective and neuromodulatory effect. A variety of studies on AK and related enzymes have been carried out to understand its mechanism of action and to identify potent inhibitors of the enzyme with therapeutic potential. These studies include (i) Relationship of AK-mu3A gene alterations to Herman-Pudlak Syndrome; (ii) Molecular characterization of human cell mutants affected in AK; (iii) Understanding the molecular mechanism by which phosphorous and other pentavalent ions stimulate AK activity; Structural studies on AK have also been carried out to determine the binding site of penatvalent ions; (iv) Screening of various chemical libraries to identify different novel inhibitors of AK, and to characterize the mechanism of action of these inhibitors; (v) Biochemical studies on other enzymes that are similar to AK also show pentavalent ion dependency.
This area of research is no longer being pursued in our lab. For our work in this area, please see publications.
Sequence characteristics of the short and long isoforms of AdK. The amino acid sequences of these two isoforms in human, mouse and rat are identical except for the sequence at the N-terminus region (NTS). The NTS of AdK-long in these species is highly conserved with complete conservation of the central basic cluster (PKPKKLK).
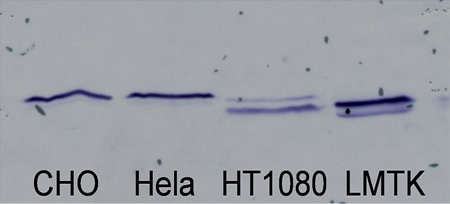
Western blot analysis of cell extracts from different cell lines. About 40 μg of total protein from various cell lines: CHO (Chinese hamster ovary), HeLa (a human epithelial cell line derived from cervix), HT-1080 (a human epithelial cell line from connective tissue), and LM (TK−)(a fibroblastic cell line of connective tissue) was loaded onto 12% SDS–PAGE gels. After electrophoresis and transfer of proteins to nitrocellulose membrane, the blot was reacted with 1:1000 dilution of rabbit polyclonal antibody to AdK. The blot was treated with a secondary antibody conjugated to horseradish peroxidase and the cross-reactive bands were visualized by chemiluminescence.
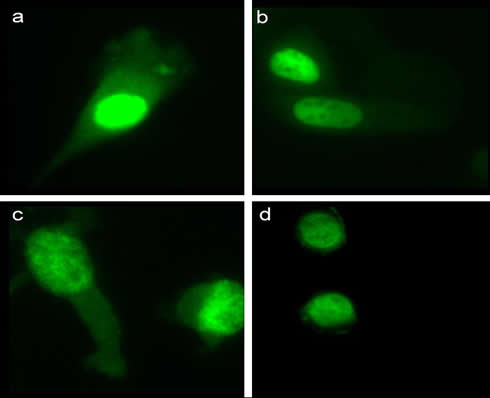
Differential expression and subcellular localization of the two AdK isoforms in mammalian cell lines. Immunofluorescence localization of AdK in the (a) HT-1080, (b) HeLa, (c) LM (TK−) and (d) CHO cell lines using the AdK-antibody. In HeLa and CHO cells, which contain only the long isoform of AdK, immunofluorescence labeling was mainly observed in the nucleus.
